


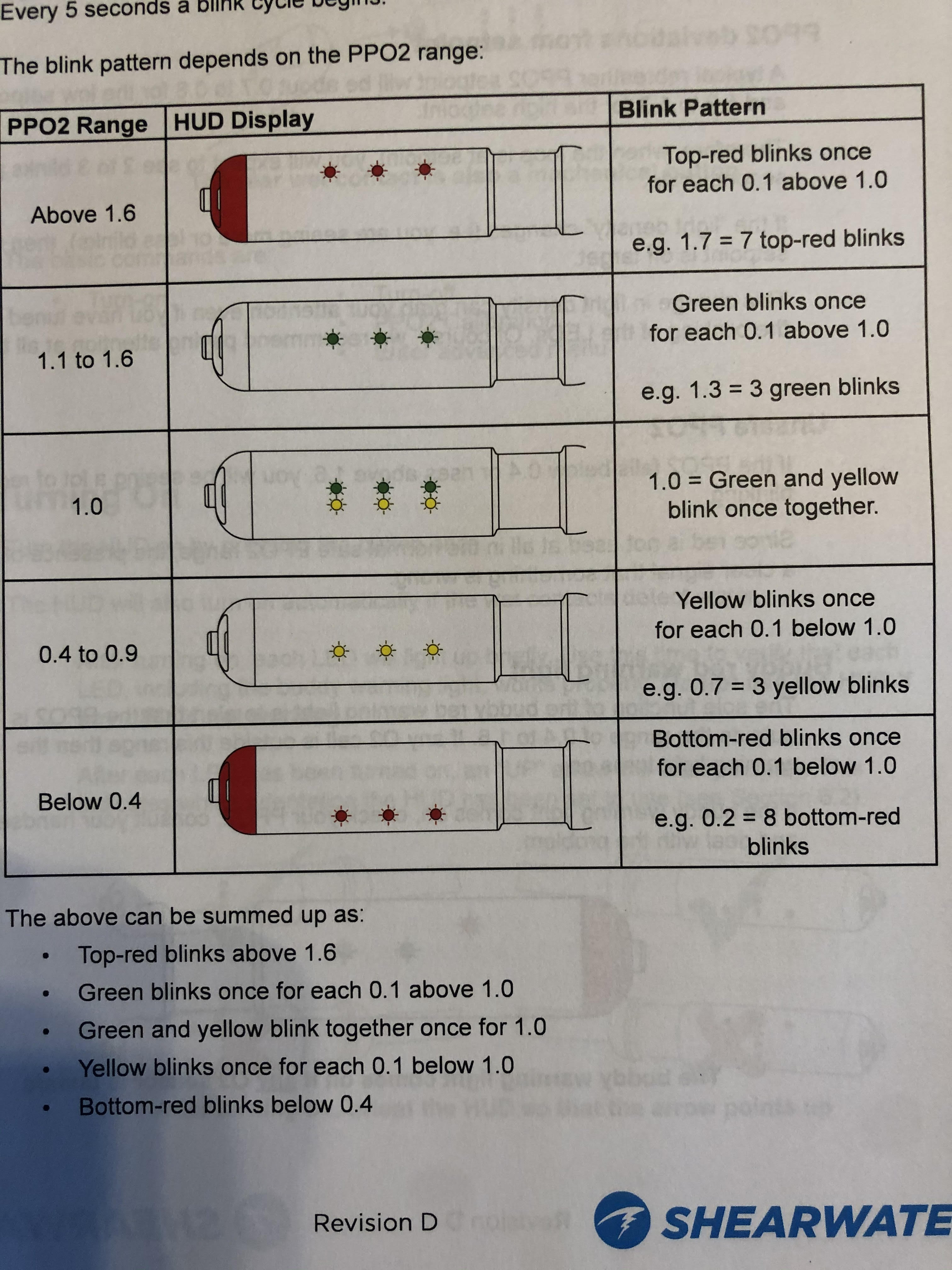
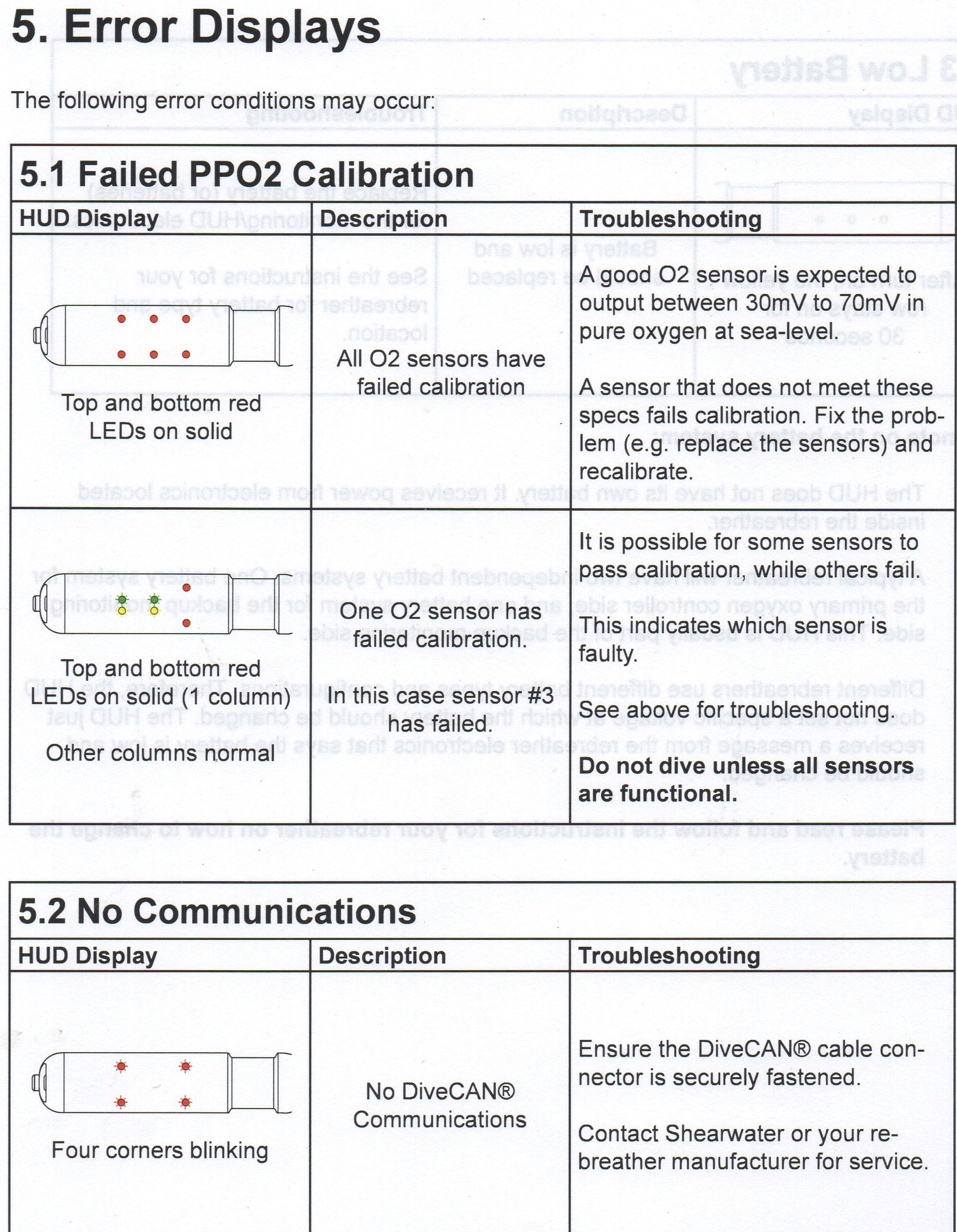






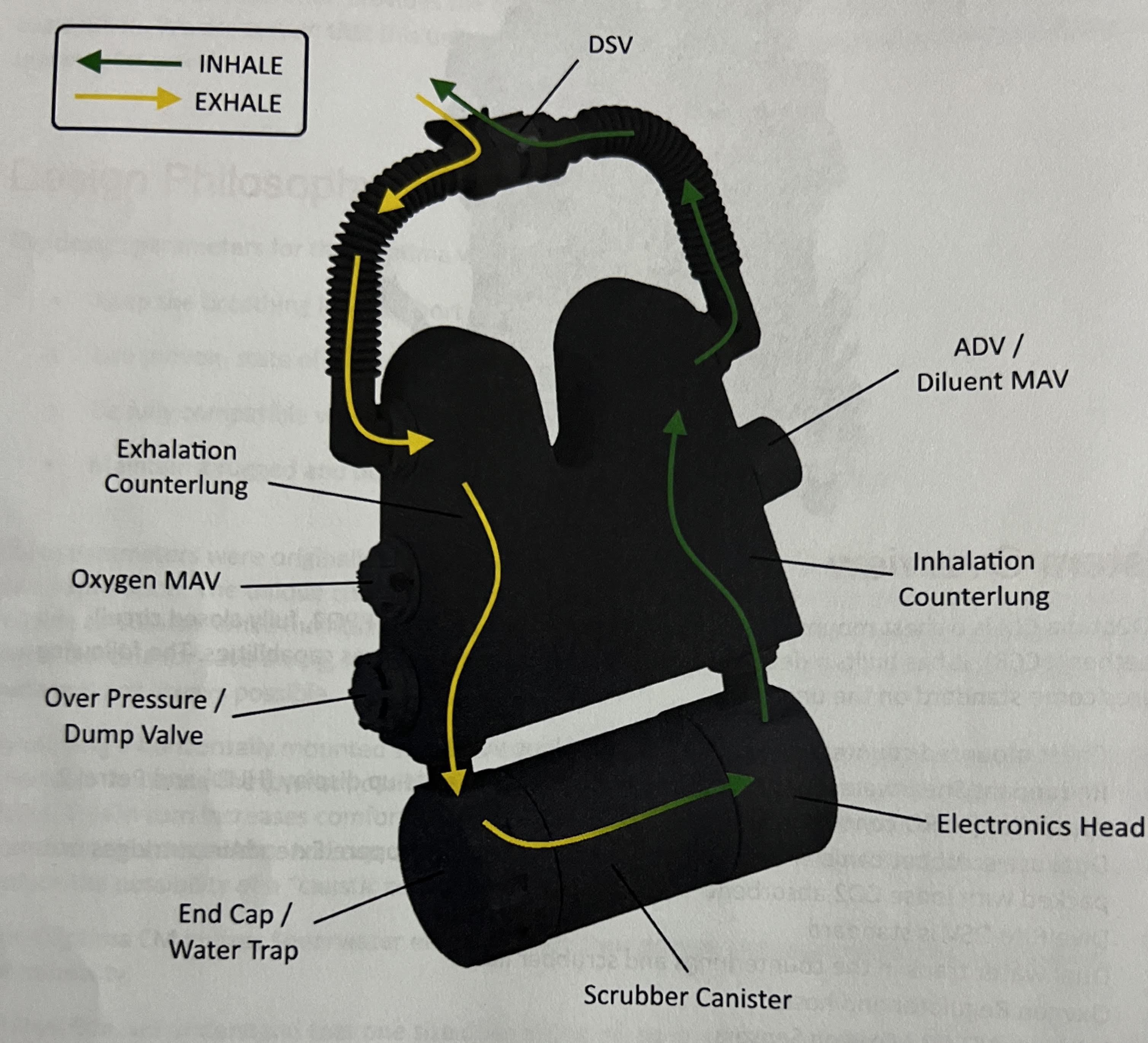
| The Loop Gases Explained | CCR Setpoints |
| Servicing the DSV | Gas Density, WOB and CO2 |
| Gradient Factors |
At rest, CO2 production may be as low as .3 to .5 LPM.
At hard work it may be about 4 LPM.
1.0 LPM CO2 production is more realistic for what we are doing.
For most scrubbers output of CO2 stays at zero until 60 to 80% of the absorbent is used up.
Almost all standards for testing of scrubber endurance, for instance the European standard EN 14143, U.S. Navy, the U.S. National Institute of Occupational Safety and Health (NIOSH) agree that the cut-off level for pCO2 should be 0.5 kPa. There are two reasons:
1) how people react to inhaled CO2,
2) the rapid rise in pCO2 leaving the scrubber after it reaches this level.
.5 kPa = .072 psi, or .005 bar
Workload. How hard you work during a dive has the biggest effect on scrubber endurance: you may be waiting quietly for a fish to be visible for a photo or swimming very hard upstream in a high flow cave. Your breathing may vary from 10 to 15 L/min to over 75 L/min. The CO2 load that the scrubber has to manage will change with it. A 5-fold increase in work rate will not just reduce the scrubber endurance time by a factor of 5, but may actually decrease it by a factor of 10.
The water temperature is another big factor. If you are diving in warm water, the absorbent will be warmer than it would be in cold water. Generally the absorbent is better at removing the CO2 when it is warm. For instance, diving in 15 °C (59 °F) water may increase the endurance by 50% compared to 4 °C (40 F)water where the stated endurance was most likely determined in laboratory testing. Diving in 30 °C (86 °F) water may increase the endurance by another 25 to 40%.
Diving depth. The depth has an effect too. An increasing depth tends to decrease the scrubber endurance. However, the size of this decrease is hard to specify because it depends on the rebreather design and type of absorbent used. The likely reason for this decreased endurance is that increasing depth means denser gas which is better at cooling the absorbent.
Absorbent packaging. Most absorbent is sold in a granular form. The granule size is generally rated on a scale from 4 to 12, where 4 is a large granule and 12 is a small one. Often the absorbent comes in a range of sizes, for instance 4-8 or 8-12. Some pre-packaged canisters have other numbers. One manufacturer sells a product that consists of powdered absorbent mixed with a polymer, shaped with defined ridges and then rolled up. Generally, smaller granules have more surface area and tend to be more efficient than the larger granules, but increase the breathing resistance. A rebreather manufacturer has to decide on the trade-off between scrubber efficiency and breathing resistance when deciding which absorbents may be used in a particular rebreather. There is a similar trade-off when deciding on the shape of the ridges of the rolled-up type of absorbent.
Gas density / depth. With increasing depth, the gas passing through the scrubber gets denser and therefore cools the absorbent more, making it less efficient. At very great depths the rate of gas diffusion decreases. Simply put, other gas molecules are in the way of the CO2 on its way to the absorbent.
Before changing the absorbent, some divers will use their rebreathers much longer than the stated scrubber endurance. This can be a very tempting, but a dangerous way of diving because the safety margins are gone. When the scrubber is nearing the end of its endurance it is not capable of challenging use. An almost used up scrubber can’t handle a challenge
For civilian rebreather diving, the European standard EN 14143 specifies how a scrubber’s endurance time must be tested. The entire rebreather is submerged in water at a temperature of 40°F. A breathing simulator is set at a minute ventilation of 40 L/min with a CO2 production of 1.6 L/min.
Summary
The scrubber endurance time varies greatly due to many factors, primarily the diver’s workload and the water temperature. Therefore, the scrubber endurance time is given during conditions where the absorbent efficiency is expected to be low, i.e. at a moderate workload, in cold water and at the maximum depth that the rebreather will be approved for. Use only the absorbent that the rebreather manufacturer has recommended.
For every liter of O2 metabolized you make .92 liters of CO2.
For a 19FT3 O2 bottle - it has 537 liters of O2 (19 X 28.3 = 537.7) so 537 X .92 = 494 liters CO2 produced from a full 19FT3 bottle of O2.
An Extendaire cartridge will scrub 300 liters of CO2. So if you produce 1 LPM of CO2 an Extendaire cartridge will last 326 minutes, or 5.4 hours. If you use the .92 production rate.







Oxygen Metabolism varies with workload
At Rest 0.3 to 0.5 Litres/minute (LPM)
Light to Moderate Work 1.5 LPM
Max work 3-3.5 LPM
1 cubic foot = 28.3 litres
one dry liter=.035 dry FT3
Figure bottle size in metric where 1FT3 = 28.3 Liters
19FT3 O2 bottle X 28.3 = 537.7 Litres of gas
Then figure Oxygen metabolic rate = 1 LPM nominally
Then figure cylinder duration: 537 L / 1 LPM = 537 minutes, 8.9 hours
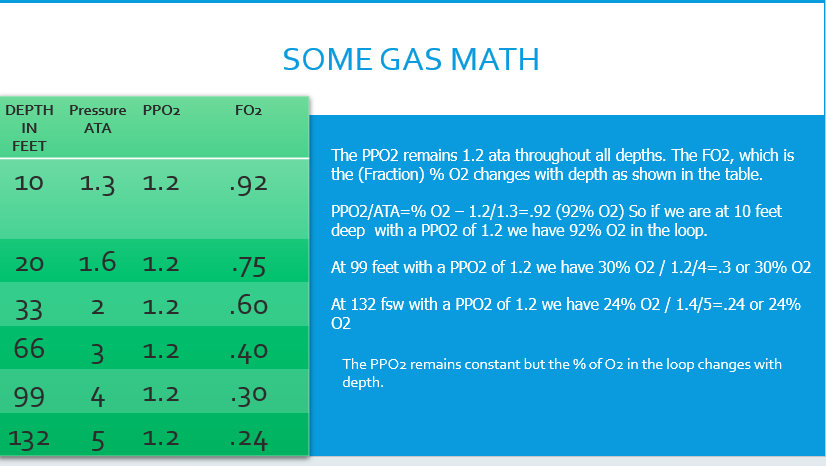
| Depth Feet | Depth Meters | Pressure-Bar | PO2 | FO2 |
|---|---|---|---|---|
| 10 | 3 | 1.3 | 1.2 | .92 |
| 20 | 6 | 1.6 | 1.2 | .75 |
| 33 | 10 | 2 | 1.2 | .60 |
| 66 | 20 | 3 | 1.2 | .40 |
| 99 | 30 | 4 | 1.2 | .30 |
| 132 | 40 | 5 | 1.2 | .24 |
| 165 | 50 | 6 | 1.2 | .20 |
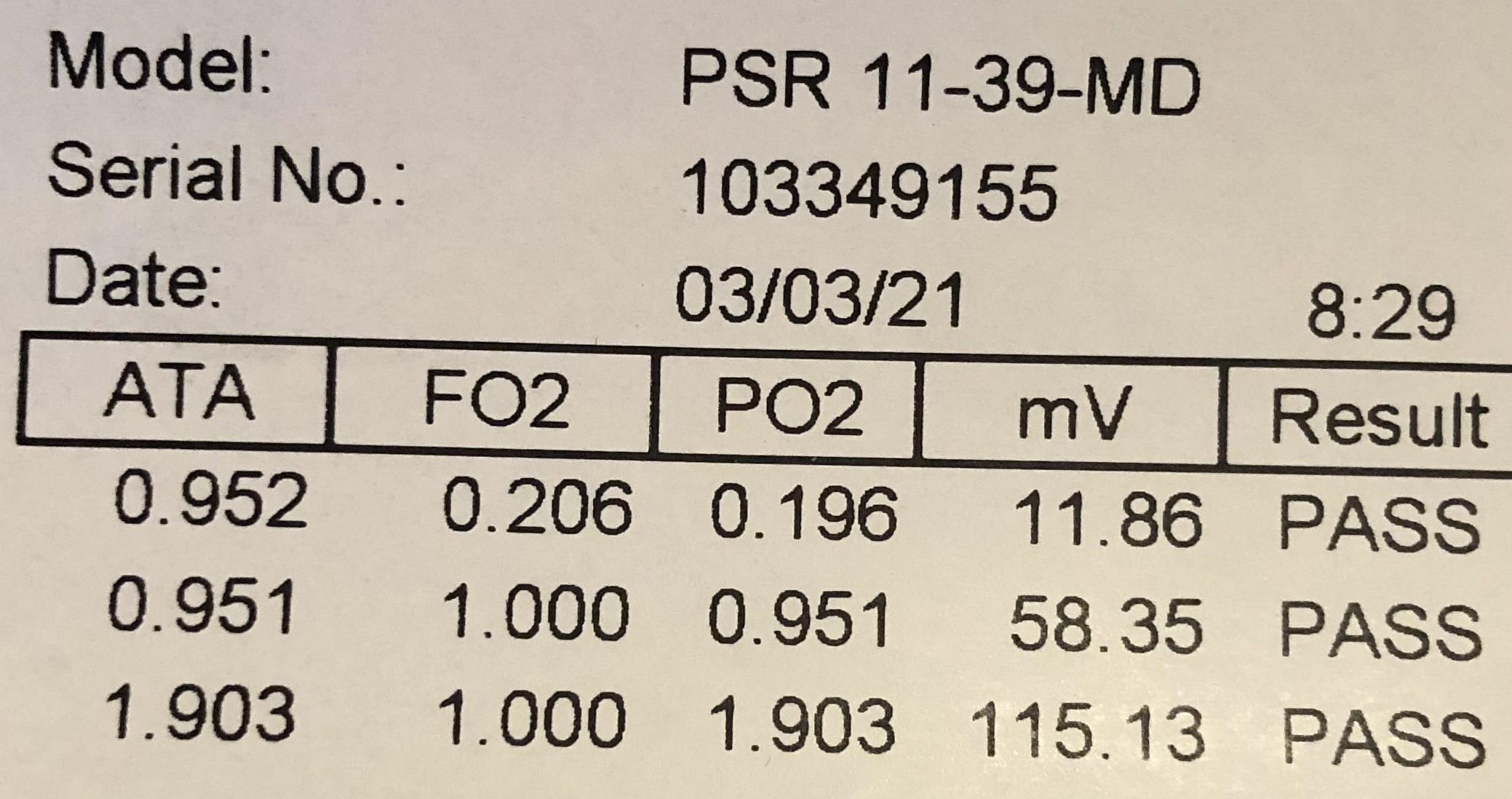
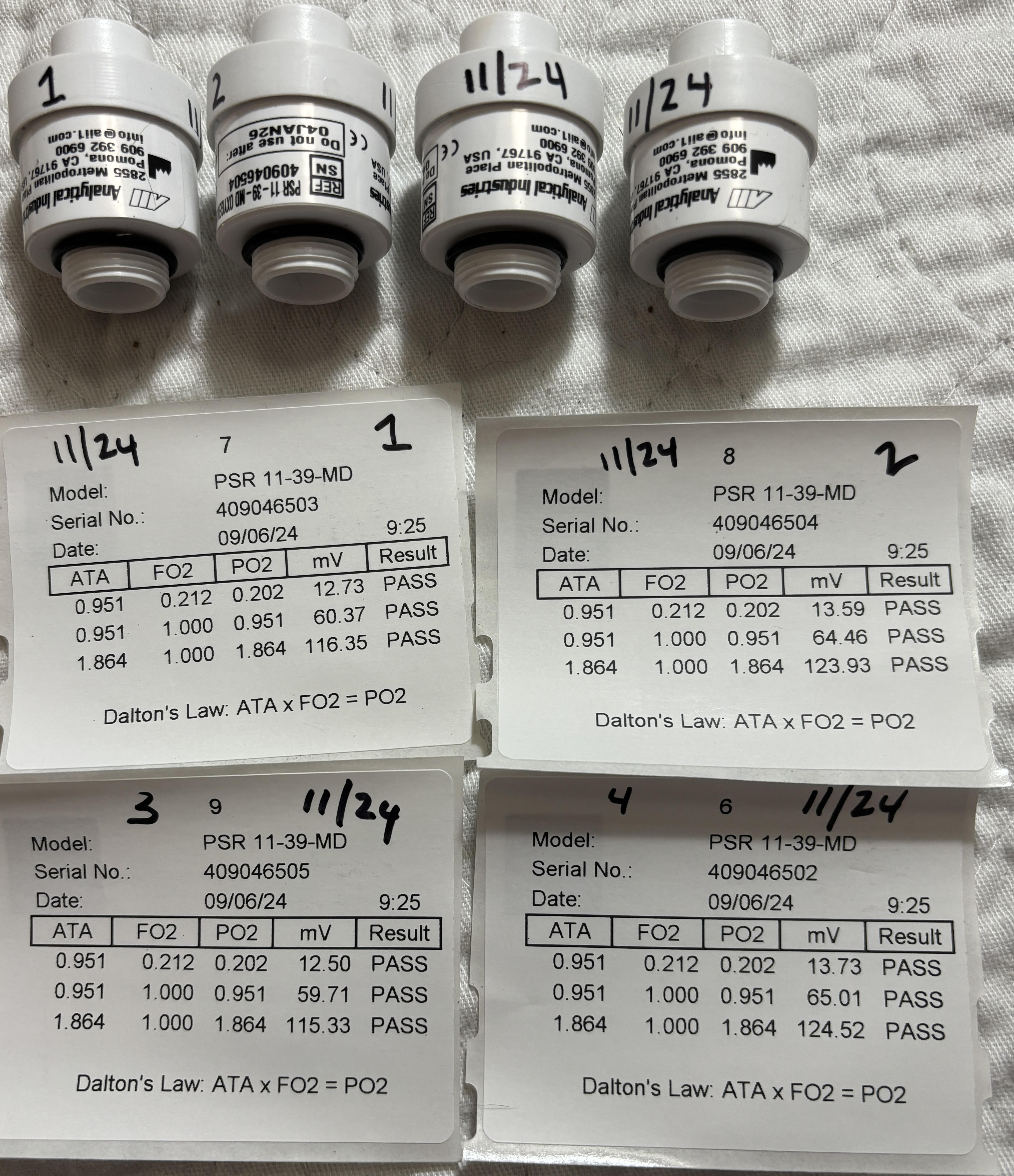
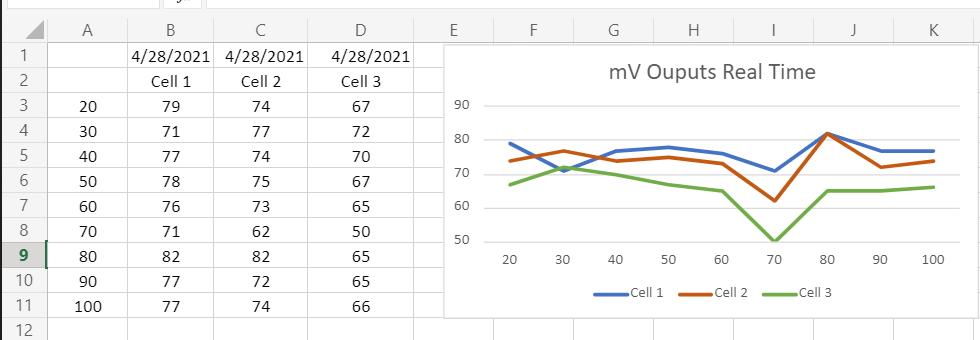
The image above is from a cell showing the test results of that cell before it was shipped from the manufacturer.
The cell was tested in pure air at the surface with a resulting mV output of 11.86.
The cell was tested at the surface in pure O2 with a resulting mV output of 58.35.
The cell was also tested at 1.9 ata in pure O2 with a resulting mV output of 115.13.
21 is to 10 as 98 is to X --> 21/10:98/X -->X=46.6mV
The above formula/ratio is one way to predict the expected output in mV of an oxygen cell
based on the gas in the head ASSUMING the cell will output 10mV in air.
Where 21=FO2, 10=mV output in air at 1 ata, 98=FO2, and solving for X which is the predicted mV output for that FO2.
1 atm / .21 atm = 4.76
eg. Cell 1 reads 11 mV in Air, Cell 2 reads 10.1 mV in Air, Cell 3 reads 12.5 mV in Air
To predict what each cell will read in 100% O2
11 X 4.76 = 52.36 mV for Cell 1.
10.1 X 4.76 = 48.0 for Cell 2.
12.5 X 4.76 = 59.5 for Cell 3.
Sensors should read 10-14mV in Air and 50-62 mV in pure O2 at 1 ata.
Oxygen sensors are the Achilles’ heel of all modern rebreathers. Sensors have a finite life span and should be considered consumable items. They are prone to failure and it is important to know how and why they can fail.
Oxygen sensors are essentially oxygen powered galvanic fuel cells. They are electrochemical devices that produce a weak electric current in the presence of oxygen. Over time, the chemicals are slowly consumed and the sensor becomes increasingly unreliable.
A new sensor will have a linear response to the partial pressure of oxygen throughout the normal operating range used in rebreather diving. However, as they age, their output becomes increasingly non-linear at higher partial pressures of oxygen. This is commonly referred to as “current limiting.” The problem with this is that a sensor may appear to be acting normally, but display a lower PPO2 than what is actually in the breathing loop. If the diver (or electronic controller) is using this value to determine oxygen injection it can quickly lead to a hyperoxic breathing loop.
This is the primary reason for having multiple oxygen sensors. It allows the diver (or controller) to look at all sensor values simultaneously and easily determine if one is giving a different reading from the rest. If a discrepancy occurs, the diver has several options available to determine which sensors are reading correctly and which should be ignored. These procedures will be covered in your training. Any sensors that are suspected of being current limited must be replaced before diving the unit.
The response time of aging sensors will also slowly increase. If you notice one or more sensors responding slowly to changes in PPO2 compared to the other sensors, they should be replaced.
Many rebreather fatalities have occurred because of using old sensors. Dive Rite recommends that sensors be used for no more than 12 months. The clock starts ticking as soon as the sensor is removed from the original packaging. The 12 month limit is independent of the number of dives on the unit. Even if the rebreather is stored and unused for a long period of time, the sensors should still be replaced prior to diving if they have been installed for more than 12 months.
Even unused sensors still in their original packaging have a limited shelf life. Dive Rite uses Analytical Industries, Inc., sensors which have a “Sell by” date of 4 months after manufacture and a “Do not use after” date of 16 months after manufacture. Even if the sensor has been in use for less than 12 months and seems to be working correctly, it should be discarded and replaced once the “Do not use after” date is reached.
Because of the limited shelf life, it is recommended that you do not keep a large stock of “backup” sensors. It is much better to purchase fresh sensors as they are needed.
There are different theories about the best replacement schedule for sensors. Many divers opt to replace all four sensors at once during the annual service every year. An alternative replacement schedule is to replace one sensor every 3 months. The sensor that is replaced should either be the oldest of the set, or alternatively the one that is the slowest to respond to PPO2 changes. This method has the advantage that each sensor will be from a different manufacturing batch. Although rare, it is possible for an entire batch of sensors to be defective and fail prematurely. The age of the sensors will also be staggered with this method and will lessen the chance that multiple sensors will fail at the same time.
Replacing sensors in a timely manner will mitigate a lot of the potential issues, however it is possible for sensors to fail before their expiration date is reached. Even brand new sensors can fail. It is always best to keep a close eye on your HUD and controller for any discrepancies and replace any suspect sensors immediately.
Sensors should not be removed from the unit for storage in between dives. This practice is unnecessary and can lead to damaged sensors and increased wear and tear on the wiring harness. Sensors should never be frozen, vacuum sealed, or stored in inert gas or desiccant in an attempt to increase their life span. Any of these practices will likely damage the sensor. Sensors, along with the rest of the rebreather, are best stored in a cool (but not freezing), dry location out of direct sunlight.
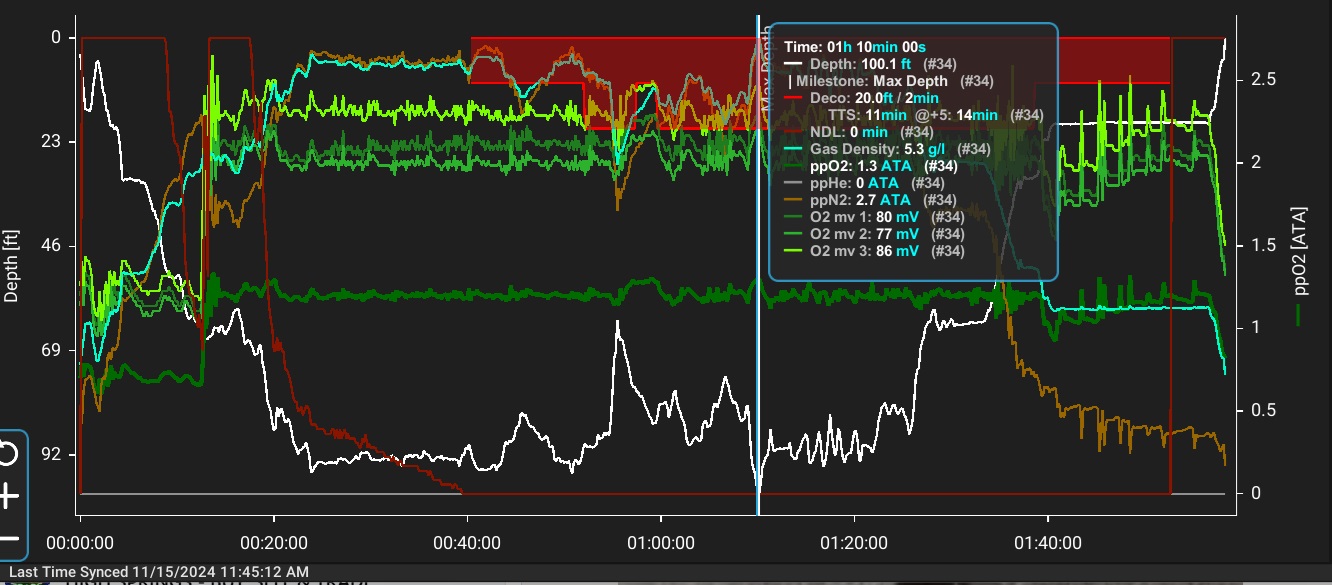
Gradient factors (GF) modify M-values (and consequently allowed gas supersaturation) to a fraction of the difference between ambient pressure and the original M-value. Thus, GF 80 modifies the M-value to 80% of the difference between ambient pressure and the original M-value. Typical proprietary implementations of the GF method require the diver to select two gradient factors: GF low modifies the M-values for the deepest decompression stop, and GF high modifies the M-value for surfacing (often designated as GF low/high, e.g. GF 30/80). The algorithm then interpolates a series of modified M-values in between these two user-specified points. If the GF low is set less than 100%, this forces deeper stops to limit supersaturation in the fast tissues early in the ascent, and setting the GF high to less than 100% will produce longer, shallower stops to reduce supersaturation in the slower tissues in the latter phase of the ascent


725 psi = 50 bar
3000 psi = 204 bar
LP 85 = 12 liter
LP 104 = 16 liters
AL80 = 11 liters
LP 121 = 18 liter
LP 95 = 15 liter
28.3 liters = 1 cubic foot









If you are trained on this rebreather, or any rebreather for that matter it should be your PRIMARY scuba unit.
Do not try to save money on cells or sorb. Bad cells and/or used up sorb will kill you and kill you quickly.
Keep it in muscle memory, keep it familiar. Dive it frequently.
DO NOT BE CHEAP Do not start a dive withn a known failure, abort a dive when a failure occurs.
Replace O2 cells regularly and particularly when one is not linear or acting like it should.
Track your O2 cells mV on a piece of paper or Excel spreadsheet.
Leave the cells in the head. Do not refrigerate or store them in an inert gas. You can best extend their life by leaving them exposed to air.
Store the unit in a cool, dry place.

